Featured
Bf3 Lewis Acid Or Base
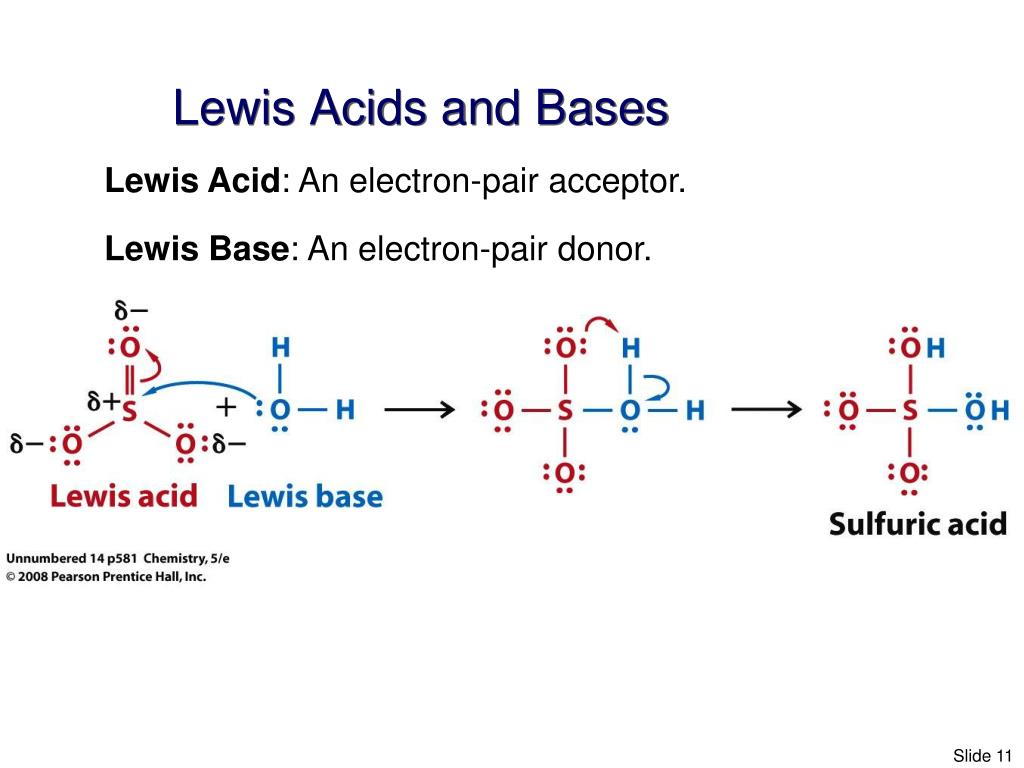
Bf3 Lewis Acid Or Base. If you draw out the lewis structure for bf3, you will get the boron in the center with the three fluorine's coming off of it. Many students are also confused that boron trifluoride (bf3) is an acid or base?.
> we would expect bf_3 to be stronger, because f is more electronegative than cl. Bf3 as a lewis acid. Hardness and softness of any species can be explained by pearson hsab principle.
What Is Lewis Acid And Lewis Base?
What type of base is bf3? Many students are also confused that boron trifluoride (bf3) is an acid or base?. Bcl_3 is the stronger lewis acid.
So, To Complete The Octet Boron Accepts Two Lone.
Is bf3 bcl3 or lewis acid better? Fluorine is more electronegative than hydrogen, so due to inductive effects, the electron density around boron. In the case of bf 3, boron forms three covalent bonds with three hydrogens but the octet of boron is still incomplete.
>> Acids, Bases And Salts.
Bf3 and bh3 both accept electrons at the boron atom. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Boron trifluoride shows very interesting behavior in the different states of water.
Hardness And Softness Of Any Species Can Be Explained By Pearson Hsab Principle.
All of the fluorine's have a full. A lewis acid can accept a pair of electrons from a lewis. Whereas a lewis acid can accept a pair of electrons from.
Normally Lewis Acids Are Species With Vacant.
Chemists explain this unexpected result by an. The gas of bf3 is very toxic, which can damage the organism, such as animal, plant, and bacterium. Correct option is a) lewis acid is a chemical species that reacts with a lewis base to form a lewis adduct.
Comments
Post a Comment